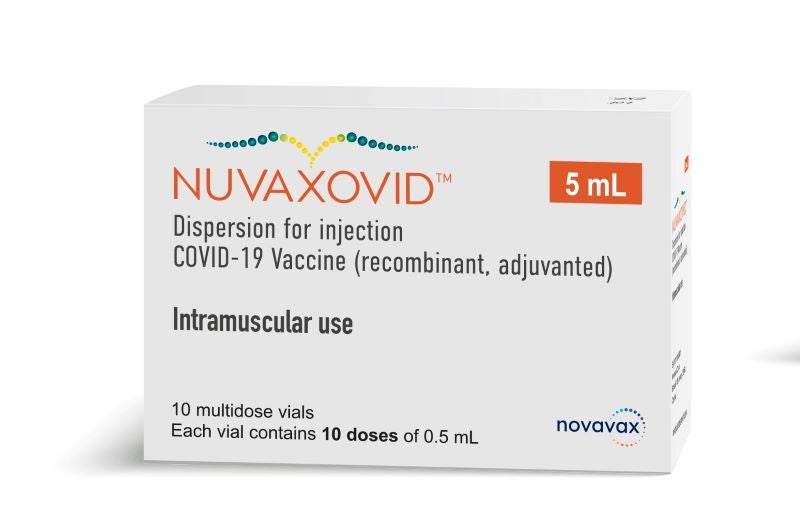
Nuvaxovid
Det eftersom att data. Nuvaxovid dispersion for injection.

Ministry Of Health Singapore On Instagram Registration For The Nuvaxovid Vaccine By Novavax Has Begun Individuals Aged 18 Years And Above May Receive The Vaccine For Their Primary
Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista.

. Nuvaxovid-rokote sopii lähes kaikille aikuisille. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022.
Nuvaxovid is the first protein-based COVID-19 vaccine granted. This is a multidose vial. After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further.
Qualitative and quantitative composition. -- Adolescents ages 12 to 17 can now receive the Novavax COVID-19 vaccine the fourth vaccine to be authorized for the prevention of coronavirus. Nuvaxovid COVID-19 vaccines are available for use in the United Kingdom as of September 27 2022.
HSAs assessment is that although the. COVID-19 Vaccine recombinant adjuvanted 2. The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19.
Adults who havent gotten any COVID-19 shots yet should consider a new option from Novavax a more traditional kind of vaccine health officials said. The Summary of Product Characteristics is a description of a. On December 20 2021 the.
Beslutet är temporärt och gäller från. As of January 2022 approximately 300 million people worldwide have been infected with the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 that causes coronavirus. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older. Enligt testerna skulle det ju vara säkrare än Pfizer hur kan det komma sig att Pfizer används. As such HSA will be monitoring the incidence rate of pericarditis or inflammation of the outer lining of the heart and myocarditis.
The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Nuvaxovid contains a version of a protein found on the.
The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19. 2 hours agoPublicerad idag 0702. Vaccinationer med Nuvaxovid pausas för personer 30 år och yngre Folkhälsomyndigheten.
The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the European Medicines Agency. Rokotteesta ei myöskään ole haittaa vaikka. About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach.
About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. Name of the medicinal product. The addition of the saponin-based.

Covid Vaccine Novavax Requests Who To Expand Emergency Use Listing Of Nuvaxovid For Adolescents Aged 12
Faq What You Need To Know About Novavax S Non Mrna Covid 19 Vaccine Nuvaxovid Cna

What To Know About The New Novavax Vaccine For Covid 19 The Hill
Ema Chmp Recommends Cma For Novavax S Covid 19 Vaccine As Booster
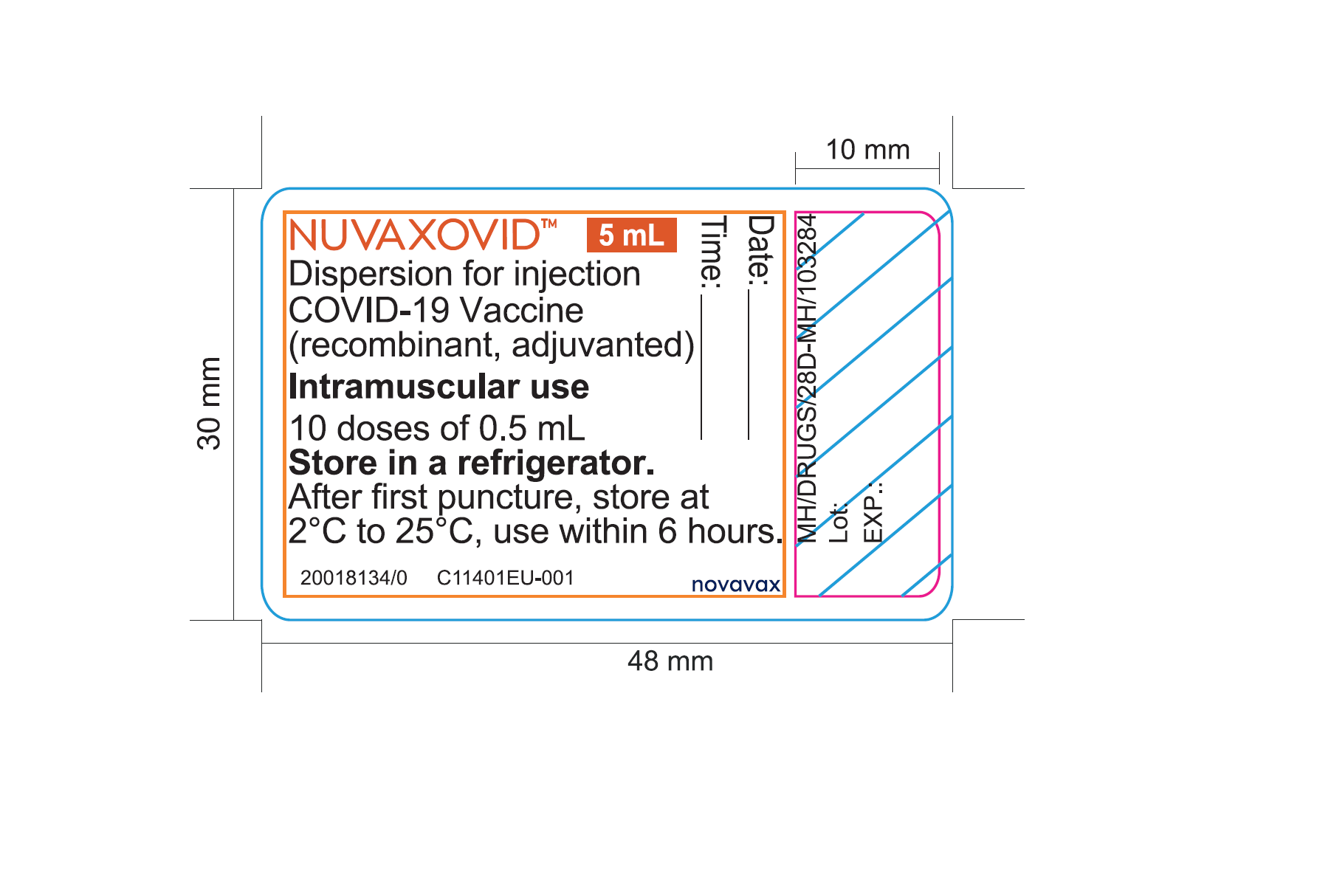
Distribution Of Nuvaxovid With English Only Vial And Carton Labels Canada Ca

Fda Advisers Overwhelmingly Endorse Novavax Covid 19 Vaccine Ars Technica

Infomesen Long Covid 19 Nuvaxovid Novavax Vaksin Australian Government Department Of Health And Aged Care

Msf Canada On Twitter Msf Comment On Canadian Approval Of The Novavax Nuvaxovid Vaccine For Covid19 And Canada S Role In Global Vaccineequity Https T Co Vkdoxh9sjy Twitter

Nuvaxovid Fifth Vaccine Against Covid Authorised In Eu Euractiv Com

Vaccino Nuvaxovid Novavax Come Funziona Effetti Collaterali
Dossier Coronavirus Sars Cov 2 And Covid 19 Suspected Adverse Reactions Reported In The First Three Months Since Start Of Vaccination With Nuvaxovid Paul Ehrlich Institut

Canadian Trademarks Details Nuvaxovid 2163962 Canadian Trademarks Database Intellectual Property And Copyright Canadian Intellectual Property Office Innovation Science And Economic Development Canada

Novavax Nvx Cov2373 Nuvaxovid In Europe And Australia Covovax In India And The Philippines Tak 019 In Japan

Novavax Announces Shipments Of Its Covid 19 Vaccine To European Union Member States Feb 23 2022

European Union Authorizes Novavax Booster

Coronavirus Q A On The Nuvaxovid Covid 19 Vaccine Cyprus Mail
Novavax S Covid 19 Vaccine Nuvaxovid Gets Conditional Approval In Switzerland Seeking Alpha

